Electrochemical study of the promoting effect of Fe on oxygen evolution at thin ‘NiFe–Bi’ films and the inhibiting effect of Al in borate electrolyte†
Abstract
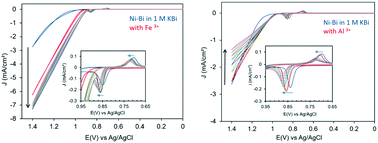
In this study, we investigated the electrochemical effects of Fe co-deposition in thin Ni oxo/hydroxo films in borate, termed ‘NiFe–Bi’, electrodeposited from a Ni : Fe ratio of 9 : 1, 6 : 4, and 4 : 6 in solution. The NiFe–Bi films were investigated as-deposited and after anodic conditioning, and compared to Ni–Bi without intentional Fe doping at Ni(OH)2 loading from a submonolayer to 10 layers. Fe co-deposition enhanced the oxygen evolution reaction (OER) of as-deposited NiFe–Bi films relative to as-deposited Ni–Bi; however, anodic biasing was still required to maximize catalysis. The Ni(OH)2/NiOOH redox peaks of NiFe–Bi deposited from 6 : 4 and 4 : 6 Ni : Fe were more cathodic and more reversible than Ni–Bi peaks. Anodic conditioning caused the Ni(OH)2/NiOOH redox peaks of Ni–Bi and NiFe–Bi to shift anodically but without narrowing of peak separation, different from the effects of Fe co-deposition. After anodic conditioning, the turnover frequency (TOF) for OER per Ni center for Ni–Bi and NiFe–Bi was more proportional to the Ni content within the first linear Tafel region, with a promoting effect of Fe, and the films exhibited similar Tafel slopes of 37–42 mV dec−1. With increasing overpotential however, the TOF increased more significantly at high Fe : Ni ratio and was not proportional to the Ni content, and the Tafel slopes varied while notably decreasing for some NiFe–Bi films. Electrochemical results can support a Ni active site at low overpotential, but point to possibly different roles of Fe at low versus high potential. In addition, the effect of adding Fe3+ and Al3+ to the electrolyte was studied after deposition of Ni–Bi. Adding Fe resulted in reaching almost maximum activity in a single potential scan, confirming the promoting role of Fe in Ni–Bi and providing a quick alternative to applying anodic bias for hours to increase activity, while Al in the electrolyte poisoned OER catalysis at Ni–Bi and at NiFe–Bi. In the presence of both Fe and Al ions in solution however, with their opposite effects on the OER, the Ni(OH)2/NiOOH redox peaks shifted anodically with potential scanning.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...