One-pot construction of Fe/ZSM-5 zeolites for the selective catalytic reduction of nitrogen oxides by ammonia†
Abstract
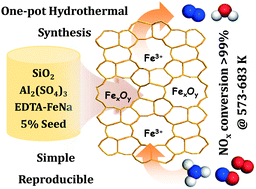
A direct hydrothermal synthesis approach to Fe/ZSM-5 zeolites was developed by using a ferric complex, i.e. ethylenediaminetetraacetic acid ferric sodium (EDTA-FeNa), as both an iron source and a structure-directing agent. During the hydrothermal synthesis, EDTA-FeNa complexes were encapsulated within zeolite channels of ZSM-5 and they underwent transformation to highly dispersed extraframework iron species, i.e. isolated ferric ions and oligomeric FexOy clusters, upon calcination removal of organic species. The as-prepared Fe/ZSM-5 zeolites could be established as bi-functional catalysts containing both acid sites and iron sites. As expected, the as-prepared Fe/ZSM-5 zeolites exhibited remarkable catalytic activity in the selective reduction of nitrogen oxides by ammonia (NH3-SCR), with a nitrogen oxide conversion of >99% in a wide temperature range of 573–693 K under simulated industrial conditions. Meanwhile, good stability and tolerance to water vapor and sulfur dioxide could be achieved, making these Fe/ZSM-5 zeolites promising candidates for practical application. In contrast to conventional post-synthesis modification approaches to Fe/ZSM-5, the one-pot hydrothermal synthesis approach appeared to be very simple and easily reproducible, and the formation of inactive iron oxide nanoparticles can be completely avoided, which accordingly leads to high NH3-SCR activity.


 Please wait while we load your content...
Please wait while we load your content...