First-principles study of single transition metal atoms on ZnO for the water gas shift reaction
Abstract
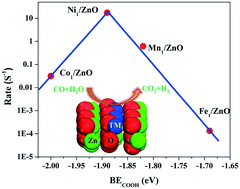
Supported single-atom catalysts have attracted increasing interest due to their high atomic efficiencies and simple structures used to establish the structure–activity relations in catalysis. In this contribution, we present a density functional theory study of ZnO supported single transition metal (TM: Mn, Fe, Co, and Ni) atoms for the water gas shift reaction (WGSR). We find that these single TM1 atoms prefer to substitute in the surface Zn lattice and exhibit promising activity for stabilizing the intermediates (i.e. CO, OH, and COOH) involved in WGSR. Meanwhile, the surface lattice O coordinated with the TM1 atoms is responsible for the hydrogen abstraction process. The formation of COOH via the association between CO and OH is the rate-limiting step in the catalytic cycle. Microkinetic modeling analysis is used to determine the activity trend, and a volcano-like plot between the calculated rates and the binding energies of COOH is obtained, suggesting that the COOH binding energy might be a good activity descriptor for catalyst screening. Among these single atoms, the single Ni1 atom exhibits the highest activity and is promising for WGSR.
- This article is part of the themed collection: Single atom catalysis


 Please wait while we load your content...
Please wait while we load your content...