Significance of isomeric reaction intermediates in the hydrogenolysis of glycerol to 1,2-propanediol with Cu-based catalysts†
Abstract
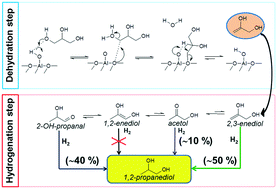
Cu/γ-Al2O3 catalysts were studied in the aqueous hydrogenolysis of glycerol to obtain 1,2-propanediol (1,2-PDO). These samples were characterized by diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy using adsorbed hydroxypropanone (acetol) in the presence of Ar or H2. Moreover, 1H nuclear magnetic resonance (1H-NMR) was used to identify the reaction products that formed when deuterated glycerol was hydrogenated in deuterated water. The DRIFT spectra revealed that enediol species are mainly stabilized at the surface of the reduced CuAl catalyst. The 1H-NMR data indicated that the reduction of the 2,3-dihydroxy-1-propene (2,3-enediol) isomer is kinetically favored to form 1,2-PDO, whereas the 1,2-dihydroxy-1-propene (1,2-enediol) species needs to first be converted to a 2-hydroxypropanal (2-OH-propanal) isomer. Therefore, the stabilization and reducibility of these enediol isomers were considered essential for the selectivity in the formation of 1,2-PDO. In addition, acetol oxidation through an interaction with surface Cu2+ or Cu+ and subsequent production of chemisorbed lactate should be avoided. A reaction mechanism with the participation of the different acetol intermediate isomers has been proposed.


 Please wait while we load your content...
Please wait while we load your content...