Tryptophan lyase (NosL): mechanistic insights into amine dehydrogenation and carboxyl fragment migration by QM/MM calculations†‡
Abstract
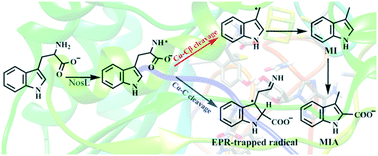
L-Tryptophan lyase (NosL), a member of the radical S-adenosyl-L-methionine (SAM)-dependent superfamily, catalyzes the conversion of L-tryptophan to 3-methylindolic acid (MIA). In this article, on the basis of the recently obtained crystal structure of NosL (PDB code 4R34) in 2014, a combined quantum mechanical/molecular mechanical (QM/MM) approach has been employed to elucidate the reaction details, involving substrate amine dehydrogenation, C–C bond cleavage and carboxyl fragment migration. Our results show that the hydrogen in the amino group of L-tryptophan is suitable for abstraction by the Ado radical, and this step corresponds to an energy barrier of 12.4 kcal mol−1. Two possible modes of C–C cleavage (path1 and path2) have been considered. The cleavage of the Cα–Cβ bond is thermodynamically more favorable than the cleavage of the Cα–C bond with their energy barriers being 7.2 and 15.0 kcal mol−1, respectively. And the easy breaking of Cα–Cβ may be attributed to the electron hole delocalization in the amine radical of the substrate. The intermediate derived from the cleavage of the Cα–C bond is calculated to be a stable species, and the cleavage of the Cα–C bond is accompanied by the migration of the ˙COO− fragment. These conclusions are basically in accordance with EPR-trapped analysis and can account for the absence of the ˙COO− fragment. The shunt product (3-methylindole) is obtained from the cleavage of the Cα–Cβ bond with an energy barrier of 19.5 kcal mol−1. However, the rate limiting step is the formation of 3-methylindolic acid (MIA), which corresponds to an energy barrier of 26.9 kcal mol−1. Our investigations thus give a better comprehension of the NosL reaction mechanism and may contribute to the understanding of the SAM superfamily.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...