Copper-catalysed aromatic-Finkelstein reactions with amine-based ligand systems†
Abstract
A new efficient and low-cost ligand, diethylenetriamine, has been utilised to promote the iodination of 16 different bromo-substrates via the copper catalysed Finkelstein halogen exchange reaction under mild conditions. In contrast to earlier methods, the use of inert atmosphere conditions was not required to obtain high yields and purity. Studies on the speciation of the catalyst in solution indicate rapid disproportionation of copper(I) in the presence of diethylenetriamine to give copper(0) and a bis-ligated copper(II) complex which is characterised by X-ray diffraction. This copper(II) complex was also shown to be catalytically active in the halogen exchange reaction. In contrast, no significant disproportionation was observed using dimethylethylenediamine as the ligand, and the solid-state structures of a copper(I) dimeric complex and a 2D polymeric network of copper(I) iodide tetramers are reported. The catalytic activity of diethylenetriamine and dimethylethylenediamine with both copper(I) and copper(II) salts are compared, and possible mechanistic implications discussed.
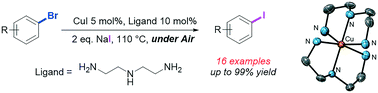


 Please wait while we load your content...
Please wait while we load your content...