Epoxidation of propene using Au/TiO2: on the difference between H2 and CO as a co-reactant†
Abstract
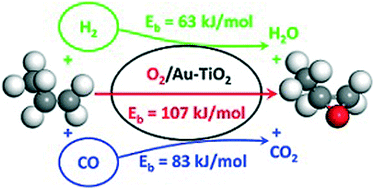
The role of the reducing gas in the direct epoxidation of propene to propene oxide (PO) using O2 over a Au/TiO2 catalyst was studied through experiments and density functional theory calculations. It was found that PO can be obtained using both H2 and CO as co-reactants. The yield of PO was much lower with CO than that with H2. The role of the oxygen atoms of the titania support was studied by quantum-chemical investigations, which show that the mechanism involving CO as a co-reactant should proceed via surface oxygen vacancies, whereas with H2 the well-accepted pathway involving OOH is favored. Steady-state isotopic transient kinetic analysis experiments demonstrate that support oxygen atoms are involved in PO formation when CO is used as the co-reactant.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...