Identification of activity trends for CO oxidation on supported transition-metal single-atom catalysts†
Abstract
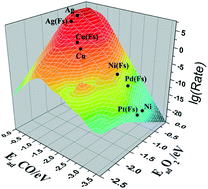
Single-atom catalysts (SACs) can have high selectivity while maximizing the efficient utilization of metal atoms and are very promising for applications in catalysis. However, the design and development of SACs cannot be effectively achieved, as little theoretical effort has been directed towards exploring the activity trends for reactions on SACs. In this work, we find that there is a Brønsted–Evans–Polanyi (BEP) linear correlation between the adsorption energies of CO and O2 and transition state energies for CO oxidation on various M/MgO and M/Fs-defect MgO SACs (M = Cu, Ag, Au, Ni, Pd, and Pt) via density functional theory (DFT) calculations. Based on the contour plot of Sabatier activity from the BEP relationships and microkinetic model, we have identified the activity trends for CO oxidation on these SACs by using the adsorption energies of CO and O2 as the activity descriptors. The theoretical calculations indicate that Ag/MgO and Ag/Fs-defect MgO exhibit better catalytic performance than the other SACs. Our results provide a general picture of the identification of the activity trends for CO oxidation on MgO-supported SACs in terms of the adsorption energies of the reactants. This approach may also lay a theoretical basis for designing new SACs for reactions other than CO oxidation.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...