Ni(ii)–N′NN′ pincer complexes catalyzed dehydrogenation of primary alcohols to carboxylic acids and H2 accompanied by alcohol etherification†‡
Abstract
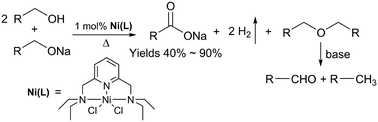
Acceptorless dehydrogenation of alcohols to carboxylic acid derivatives catalyzed by a transition metal complex is an important reaction in modern organic synthesis and catalysis, for which nickel complexes have rarely been developed. Herein we report three Ni(II) complexes bearing pyridine-based N′NN′ type pincer ligands, which catalyze the acceptorless dehydrogenation of primary alcohols to carboxylic acids under anhyrous conditions. The complex [NiCl2(L3)] 3 (L3 = 2,6-bis(diethylaminomethyl)pyridine) displays the best catalytic reactivity, catalyzing the primary alcohols to carboxylic acids and H2 in good yields (40–90%). Further investigation reveals that an unexpected alcohol etherification occurs, which gives the second oxygen atom for the formation of the carboxylic acid. Our results give a thread for the design of new nickel complexes without phosphine and N-heterocycle carbene ligands for the acceptorless oxidation of alcohols.


 Please wait while we load your content...
Please wait while we load your content...