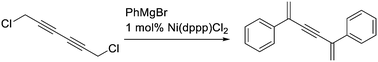
Synthesis of ene-yne-enes by nickel-catalyzed double SN2′ substitution of 1,6-dichlorohexa-2,4-diyne†
Abstract
1,6-Dichlorohexa-2,4-diyne undergoes nickel-catalyzed double substitution with aryl and alkenyl Grignard reagents to provide substituted ene-yne-enes. The reaction is formally an extension of the well-described SN2′-allylic and -propargylic substitution reactions but the mechanism is considerably more complex. The products might function as building blocks for conjugated polymers.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...