External or internal surface of H-ZSM-5 zeolite, which is more effective for the Beckmann rearrangement reaction?
Abstract
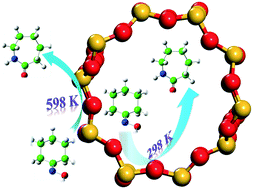
Zeolites are effective catalysts for amide formation from oxime through the Beckmann rearrangement (BR) reaction; however, debates about which surface (i.e. external or internal) is more effective for the BR reaction over zeolites still remain. In this contribution, the effective rate constants (keff) are used to quantitatively evaluate the dependence of BR reactivity on the Brønsted acid location in H-ZSM-5 zeolite. Based on our theoretical calculations, it was found that in addition to the dimension size of oxime reactants and reaction temperature, the BR reaction is strongly dependent on the location of Brønsted acid sites. For the cyclohexanone oxime rearrangement, the reaction exclusively occurs on the internal surface of ZSM-5 zeolite at room temperature, while the active sites are those located at the pore mouth or on the external surface when the reaction temperature increases to 598 K. In contrast to cyclohexanone oxime, the Brønsted acid sites on the internal surface are kinetically more effective at room temperature or 598 K for the smaller acetoxime BR reaction.


 Please wait while we load your content...
Please wait while we load your content...