Theoretical and experimental studies on the structure–property relationship of chiral N,N′-dioxide–metal catalysts probed by the carbonyl–ene reaction of isatin†
Abstract
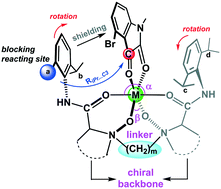
The structure and electronic properties of metallic complexes formed by coordinating chiral N,N′-dioxide ligands with different amino acid skeletons or straight-chain alkyl spacers (linkage) to Mg2+ and Ca2+ are studied at the B3LYP-D3(BJ)/6-311G**(SMD, CH2Cl2)//ONIOM (B3LYP/6-31G*: UFF) (SMD, CH2Cl2) level. The N-oxide unit in the ligand exhibits a stronger O-donor ability than the carbonyl of the amide in the formation of the chiral N,N′-dioxide–Mg(II) catalyst. Coordination of the chiral N,N′-dioxide ligand to the metal center forms a pocket-like chiral environment (“chiral pocket”), which can be characterized by four structural descriptors, which are the bite angle αO1–M–O2, the average M–O distance, the torsion angle θ2 (C1–O1⋯O2–C2) and the dihedral angle (DC1–N3–C7–C8 or DC2–N4–C9–C10). The Lewis acidity of the metal ion decreases when the number of –CH2 spacers between two N-oxide units increases from 1 to 5, which is attributed to increasing Pauli repulsion as well as deformation of fragments. The stereoselectivity of asymmetric carbonyl–ene reaction is dependent on the blocking effect of ortho-iPr of aniline on the reaction site of isatin, which could be adjusted by changing the linkage and chiral backbone as well as metal ion. An unfavorable steric arrangement in the re-face attack pathway translated into a more destabilizing activation strain of the ene substrate, enhancing the enantiodifferentiation of two competing pathways for the desired (R)-product. The counterion might change the catalytic species as well as the associated chiral pocket by taking part in coordination towards the metal center, consequently affecting the reaction mechanism and stereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...