Nanolayered manganese oxides: insights from inorganic electrochemistry†
Abstract
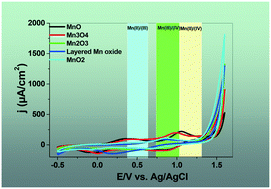
Nanolayered Mn oxides are among the important Mn-based catalysts for water oxidation. Mn(II), (III) and (IV) ions are present in the structure, and, thus, the electrochemistry of the solid is very complicated. Herein, the cyclic voltammetry of nanolayered Mn oxides in the presence of LiClO4 at pH = 6.3, under different conditions, was studied using scanning electron microscopy, transmission electron microscopy, electrochemical impedance spectroscopy, X-ray diffraction and visible spectroelectrochemistry. The scan rates, calcination temperatures and the range of the cyclic voltammetry have very important effects on the electrochemistry of nanolayered Mn oxides. The effect of the use of D2O instead of H2O on the electrochemistry of nanolayered Mn oxides was also considered. Such nanolayered Mn oxides were reported as water-oxidizing catalysts in the presence of cerium(IV) ammonium nitrate. As a next step, we studied the cyclic voltammetry of nanolayered Mn oxides under acidic conditions and in the presence of cerium(IV) ammonium nitrate.


 Please wait while we load your content...
Please wait while we load your content...