Hydroisomerization of n-hexadecane: remarkable selectivity of mesoporous silica post-synthetically modified with aluminum†‡
Abstract
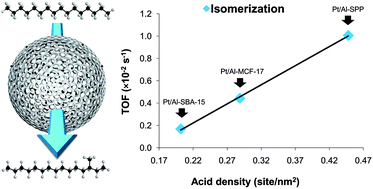
As the impact of acids on catalytically driven chemical transformations is tremendous, fundamental understanding of catalytically relevant factors is essential for the design of more efficient solid acid catalysts. In this work, we employed a post-synthetic doping method to synthesize a highly selective hydroisomerization catalyst and to demonstrate the effect of acid strength and density, catalyst microstructure, and platinum nanoparticle size on the reaction rate and selectivity. Aluminum doped mesoporous silica catalyzed gas-phase n-hexadecane isomerization with remarkably high selectivity to monobranched isomers (∼95%), producing a substantially higher amount of isomers than traditional zeolite catalysts. Mildly acidic sites generated by post-synthetic aluminum grafting were found to be the main reason for its high selectivity. The flexibility of the post-synthetic doping method enabled us to systematically explore the effect of the acid site density on the reaction rate and selectivity, which has been extremely difficult to achieve with zeolite catalysts. We found that a higher density of Brønsted acid sites leads to higher cracking of n-hexadecane presumably due to an increased surface residence time. Furthermore, regardless of pore size and microstructure, hydroisomerization turnover frequency linearly increased as a function of Brønsted acid site density. In addition to strength and density of acid sites, platinum nanoparticle size affected catalytic activity and selectivity. The smallest platinum nanoparticles produced the most effective bifunctional catalyst presumably because of higher percolation into aluminum doped mesoporous silica, generating more ‘intimate’ metallic and acidic sites. Finally, the aluminum doped silica catalyst was shown to retain its remarkable selectivity towards isomers even at increased reaction conversions.


 Please wait while we load your content...
Please wait while we load your content...