Stereoselective access to trisubstituted fluorinated alkenyl thioethers†
Abstract
We report the first copper-catalyzed olefinic ethoxy carbonyl difluoromethylation of alkenyl thioethers via direct C–H bond functionalization using BrCF2COOEt. The developed methodology allows the preparation of trisubstituted olefins with a controlled stereochemistry. A mechanistic study is reported and a radical mechanism is revealed.
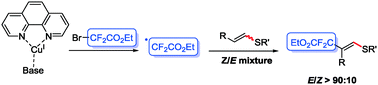


 Please wait while we load your content...
Please wait while we load your content...