Facile synthesis of CuSO4/TiO2 catalysts with superior activity and SO2 tolerance for NH3-SCR: physicochemical properties and reaction mechanism†
Abstract
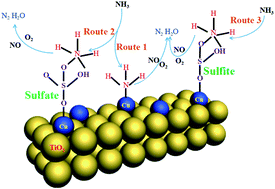
A series of CuSO4/TiO2 binary oxide catalysts with different CuSO4 loadings (0–20 wt%) were prepared by a facile solid state impregnation protocol. The physicochemical properties of the catalysts and the NO reaction route were extensively characterized via N2 adsorption/desorption, XRD, Raman, NH3-TPD, NO-TPD, H2-TPR, XPS, and in situ DRIFTS. The CuSO4 content had significant influence on catalytic activity. High CuSO4 loading favored the solid reaction between CuSO4 and TiO2, promoted the formation of sulfite and weak acid sites, and obviously increased the activity at temperatures below 340 °C. However, at a temperature higher than 340 °C, NH3 oxidation increased with CuSO4 loading and caused NOx conversion to decrease when the CuSO4 loading was higher than 10 wt%. Thus, the sample with 10 wt% CuSO4 loading presented the broadest temperature window and it also showed an excellent resistant to SO2 and H2O. Both Brønsted and Lewis acid sites were formed on the catalyst. S–OH of sulfate and sulfite were the main reason for formation of Brønsted acid sites. NH3-SCR reaction on CuSO4/TiO2 followed the Eley–Rideal mechanism: NH3 first adsorbed on Brønsted and Lewis acid sites as NH4+ and NH3, then reacted with gaseous NO and O2 to form N2 and H2O.


 Please wait while we load your content...
Please wait while we load your content...