Influence of acid–base properties of Mg-based catalysts on transesterification: role of magnesium silicate hydrate formation†
Abstract
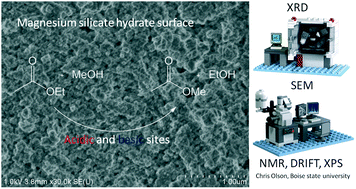
The transesterification reaction assisted through heterogeneous basic catalysis was thoroughly studied because of its importance in transforming biomass, as for biodiesel production or lactone opening. As catalysts with the strongest basic properties are not always the most efficient ones, a series of magnesium-based materials, exhibiting a large range of acido–basic properties, was investigated. Moreover, in order to compare gas and liquid phases operating conditions, a model reaction (transesterification of ethyl acetate with methanol) was chosen. It appears that gas phase transesterification (at 393 K) requires strong basic sites, whereas magnesium silicate, exhibiting moderate basicity together with acidic properties, is a very reactive catalyst in the liquid phase (at 333 K) depending on its preparation method. The set of experimental data (XRD, XPS, DRIFTS, MEB, 29Si and 25Mg NMR) demonstrated that a magnesium silicate hydrate structure (MSH) is formed at the surface of the most active silicates. It is thus concluded that different mechanisms operate under gas and liquid conditions, and that among the magnesium silicate materials, the MSH phase exhibits specific acido–basic properties beneficial to this kind of reaction.


 Please wait while we load your content...
Please wait while we load your content...