Insights into the catalytic cycle and activity of methanol-to-olefin conversion over low-silica AlPO-34 zeolites with controllable Brønsted acid density
Abstract
Low-silica AlPO-34 materials with similar crystal sizes but different Brønsted acid site densities were prepared and investigated as catalysts in methanol-to-olefin (MTO) conversion. The effect of Brønsted acid site density on catalyst activity and the dominant reaction mechanism during the MTO conversion was investigated via TGA, GC-MS, solid-state NMR spectroscopy, and in situ UV/vis spectroscopy together with the catalytic performance. For the catalysts with lower Brønsted acid site densities, the olefin-based cycle mechanism is the dominant mechanism during the MTO conversion. Long-chain alkenes, e.g., C5![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) –C6
–C6![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) alkenes, act as intermediates that are cracked to lower olefins, or are converted to dienes via hydride transfer reactions, and can also diffuse out of the cages of low-silica AlPO-34 catalysts as the products. With decreasing Brønsted acid site density or reaction temperature, the methylation route of the olefin-based cycle was found to be much more favored than the cracking route. Therefore, a higher selectivity to C5
alkenes, act as intermediates that are cracked to lower olefins, or are converted to dienes via hydride transfer reactions, and can also diffuse out of the cages of low-silica AlPO-34 catalysts as the products. With decreasing Brønsted acid site density or reaction temperature, the methylation route of the olefin-based cycle was found to be much more favored than the cracking route. Therefore, a higher selectivity to C5![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) –C6
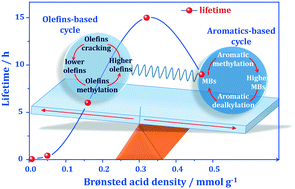
–C6![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) alkenes (∼50%) is achieved. Simultaneously, dienes are the predominant deposits occluded in the used catalysts. For catalysts with slightly higher Brønsted acid site densities, the long-chain alkenes are rapidly transformed to aromatics and, subsequently, an aromatic-based cycle mechanism contributes to the MTO conversion. Interestingly, the catalyst with the most suitable Brønsted acid site density can well balance the above-mentioned two reaction cycles accompanied by a low deactivation rate, leading to a long catalyst lifetime of up to 15 h.
alkenes (∼50%) is achieved. Simultaneously, dienes are the predominant deposits occluded in the used catalysts. For catalysts with slightly higher Brønsted acid site densities, the long-chain alkenes are rapidly transformed to aromatics and, subsequently, an aromatic-based cycle mechanism contributes to the MTO conversion. Interestingly, the catalyst with the most suitable Brønsted acid site density can well balance the above-mentioned two reaction cycles accompanied by a low deactivation rate, leading to a long catalyst lifetime of up to 15 h.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...