Mechanistic insights into the formation of butene isomers from 1-butanol in H-ZSM-5: DFT based microkinetic modelling†
Abstract
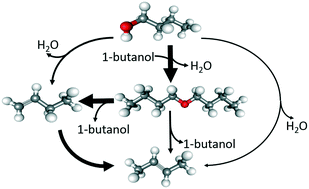
Besides being a renewable energy source, the catalytic conversion of bio-alcohols can serve as a sustainable means of producing high-value chemicals. Butenes produced by dehydration of 1-butanol could serve as building blocks for several essential compounds such as fuels and polymers. This study provides theoretical insights into the competing pathways for the formation of butene isomers (1-butene, cis/trans 2-butenes and iso-butene) during catalytic dehydration of 1-butanol in H-ZSM-5. As di-1-butyl ether (DBE) is one of the key products during low temperature dehydration of 1-butanol, a new mechanism for direct formation of trans-2-butene from DBE via E1 elimination is also envisaged along with the direct dehydration of 1-butanol to trans-2-butene. A 2-butoxide mediated stepwise mechanism and a concerted mechanism involving simultaneous protonation of the double bond by the Brønsted acid site and abstraction of the Hγ by the zeolite oxygen are considered for the double bond isomerization in H-ZSM-5. A monomolecular 2-butoxide and iso-butoxide mediated mechanism is considered for the skeletal isomerization. The transformation of 2-butoxide to iso-butoxide occurs via a π-bonded propene–methyl carbocationic transition state. DFT based microkinetic simulations show that, except for very low conversion levels where 2-butenes are produced via E1 elimination of 1-butanol from the protonated di-1-butyl ether (DBE*), the formation of 2-butenes occurs essentially via double bond isomerization mechanisms with comparable contributions of both concerted and 2-butoxide mediated stepwise mechanisms. Owing to the higher activation barrier for the skeletal isomerization, isobutene is not observed in the simulated temperature range of 450–500 K. Simulation results indicate that low reaction temperature, low site time and high butanol pressure favor production of 1-butene and DBE, while high temperature and site time and low butanol pressure favor the consecutive reactions leading to production of butene isomers.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...