Unusual deactivation of HZSM-5 zeolite in the methanol to hydrocarbon reaction†
Abstract
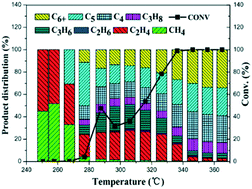
Temperature-programmed methanol to hydrocarbon (TP-MTH) reactions were performed over HZSM-5 zeolite to monitor the change of reaction performance along with reaction temperature in order to understand the mechanistic reason for the temperature influence on the reaction. With a gradual increase of reaction temperature (0.5 °C min−1), the MTH reaction could evolve from the induction period with low methanol conversion to the state with 100% methanol conversion. Four different reaction stages could be clearly observed: the initial reaction stage, the auto-catalysis reaction stage, the deactivation stage and the activity recovery stage. An unusual deactivation behavior was observed following the auto-catalysis period. Further investigations revealed that 1,2,3,5-tetraMB was the main active species during the initial autocatalytic stage and its “overloading” effect resulted in the unusual deactivation phenomenon, i.e. despite its high intrinsic reactivity, too quick formation of poorly mobile 1,2,3,5-tetraMB and lower methylbenzenes will lead to the occupation of most catalyst channels and channel intersections and cause the deactivation of HZSM-5 at low temperature. Further study demonstrated that the “overloading” effect could be alleviated or eliminated by enhancing the catalyst diffusivity or decreasing the acid site density of the zeolite catalyst.


 Please wait while we load your content...
Please wait while we load your content...