Cycloaddition reactions of enoldiazo compounds
Abstract
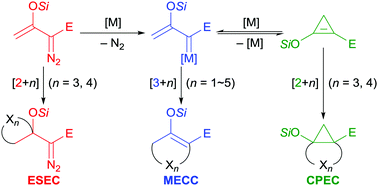
Enoldiazo esters and amides have proven to be versatile reagents for cycloaddition reactions that allow highly efficient construction of various carbocycles and heterocycles. Their versatility is exemplified by (1) [2+n]-cycloadditions (n = 3, 4) by the enol silyl ether units of enoldiazo compounds with retention of the diazo functionality to furnish α-cyclic-α-diazo compounds that are themselves subject to further transformations of the diazo functional group; (2) [3+n]-cycloadditions (n = 1–5) by metallo-enolcarbenes formed by catalytic dinitrogen extrusion from enoldiazo compounds; (3) [2+n]-cycloadditions (n = 3, 4) by donor–acceptor cyclopropenes generated in situ from enoldiazo compounds that produce cyclopropane-fused ring systems. The role of dirhodium(II) and the emergence of copper(I) catalysts are described, as are the different outcomes of reactions initiated with these catalysts. This comprehensive review on cycloaddition reactions of enoldiazo compounds, with emphasis on methodology development, mechanistic insight, and catalyst-controlled chemodivergence, aims to provide inspiration for future discoveries in the field and to catalyze the application of enoldiazo reagents by the wider synthetic community.


 Please wait while we load your content...
Please wait while we load your content...