Anchoring groups for photocatalytic water oxidation on metal oxide surfaces
Abstract
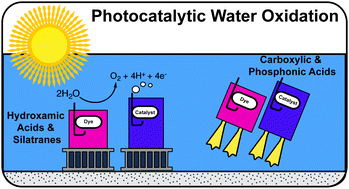
Surface anchoring groups are needed to attach molecular units to photoanodes for photocatalytic water oxidation. The anchoring group must be hydrolytically stable and oxidation resistant under a variety of pH conditions. They must sometimes be electrically conducting for efficient light-induced electron injection from a photosensitizer to a metal oxide, but other times not conducting for accumulation of oxidizing equivalents on a water-oxidation catalyst. Commonly used anchors such as carboxylic acids and phosphonic acids have limited stability in aqueous environments, leading to surface hydrolysis and loss of catalytic function. More hydrolytically stable anchors, such as silatranes and hydroxamic acids, which are oxidation resistant and stable under acidic, neutral, and basic conditions, are more suitable for photoanode applications. Hydroxamic acids can be incorporated into dye molecules to give high electron injection efficiency due to their electrical conductivity and strong electronic coupling to the metal oxide surface. In contrast, silatranes, once bound as siloxanes, have diminished electronic coupling making them useful as catalyst anchors.
- This article is part of the themed collection: Artificial Photosynthesis


 Please wait while we load your content...
Please wait while we load your content...