Photoswitchable molecules as key ingredients to drive systems away from the global thermodynamic minimum
Abstract
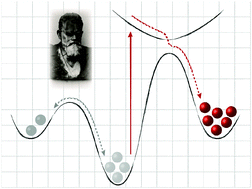
In order to perform chemical work, molecular systems have to be operated away from thermodynamic equilibrium and therefore require the input of energy. Light is perhaps the most abundant and advantageous energy source that in combination with photoswitches allows for a reversible and hence continuous stimulation of a system. In this review, we illustrate how photoswitchable molecules can be used to escape the global thermodynamic minimum by populating metastable states, from which energy can be transferred and transformed in a controlled fashion. We emphasize the unique feature of photodynamic equilibria, in which population of the states is dictated by the excitation wavelength (and not primarily by temperature), thereby avoiding microscopic reversibility since the photoreaction involves an electronically excited state. Thus, photoswitchable molecular systems can remotely be controlled with high spatial and temporal resolution and in addition their action can be fueled by light.
- This article is part of the themed collection: Chemical systems out of equilibrium


 Please wait while we load your content...
Please wait while we load your content...