The ENGases: versatile biocatalysts for the production of homogeneous N-linked glycopeptides and glycoproteins
Abstract
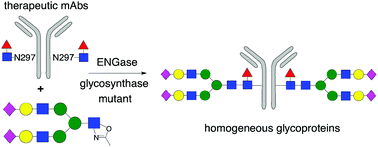
The endo-β-N-acetylglucosaminidases (ENGases) are an enzyme class (EC 3.2.1.96) produced by a range of organisms, ranging from bacteria, through fungi, to higher order species, including humans, comprising two-sub families of glycosidases which all cleave the chitobiose core of N-linked glycans. Synthetic applications of these enzymes, i.e. to catalyse the reverse of their natural hydrolytic mode of action, allow the attachment of N-glycans to a wide variety of substrates which contain an N-acetylglucosamine (GlcNAc) residue to act as an ‘acceptor’ handle. The use of N-glycan oxazolines, high energy intermediates on the hydrolytic pathway, as activated donors allows their high yielding attachment to almost any amino acid, peptide or protein that contains a GlcNAc residue as an acceptor. The synthetic effectiveness of these biocatalysts has been significantly increased by the production of mutant glycosynthases; enzymes which can still catalyse synthetic processes using oxazolines as donors, but which do not hydrolyse the reaction products. ENGase biocatalysts are now finding burgeoning application for the production of biologically active glycopeptides and glycoproteins, including therapeutic monoclonal antibodies (mAbs) for which the oligosaccharides have been remodelled to optimise effector functions.


 Please wait while we load your content...
Please wait while we load your content...