Activation volumes for cis-to-trans isomerisation reactions of azophenols: a clear mechanistic indicator?†
Abstract
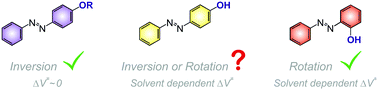
The thermal cis-to-trans isomerisation reaction of a series of hydroxy-substituted azo derivatives was studied kinetico-mechanistically as a function of temperature and pressure in order to investigate the possible role of the solvent in controlling the isomerisation mechanism, viz. inversion versus rotation. The variation of the observed first order rate constants for kinetic runs carried out at different temperatures and pressures was used to determine the thermal activation parameters ΔH‡ and ΔS‡, and the pressure activation parameter ΔV‡. In addition, some experiments with deuterated species or solvents were also performed. The reported results could be interpreted as indicative of a changeover from an inversion mechanism for non-polar solvents to a rotational mechanism for polar solvents, capable of hydrogen bonding, for some of the systems studied. However, the operation of a rotational mechanism in all studied cases can account more consistently for the data observed.


 Please wait while we load your content...
Please wait while we load your content...