Effects of different substituents of methyl 5-R-salicylates on the excited state intramolecular proton transfer process
Abstract
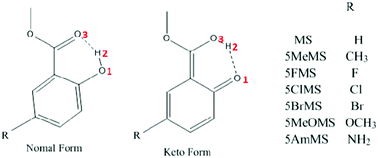
The proton transfer reaction in methyl 5-R-salicylate is found to be highly sensitive to the presence of specific substituents in resonance with the hydroxyl group, leading to different fluorescence behaviors of methyl 5-R-salicylate with different substituents (J. Catalán, J. Phys. Chem. B, 2015, 119, 2132). But a detailed survey of its reaction mechanism is lacking. In our research, the hydrogen bond strengthening behavior in excited states is affected by the different substituents that have been reported for the first time. Absorption and emission spectra calculated for the work presented here agree well with experimental results. At the same time, in order to provide a reliable description of the reaction energy profiles, we compare the barrier differences obtained using CAM-B3LYP and B3LYP methods, and we visually observe the effect of different substituents on the ESIPT reactions in methyl 5-R-salicylates by combining the potential energy curves. So the excited state intramolecular proton transfer (ESIPT) reactions in methyl 5-R-salicylate molecules are investigated in detail using density functional theory (DFT) and time dependent density functional theory (TDDFT) methods. It can be confirmed that the mobility of the intramolecular π electrons is affected by an increase in the resonant strength of the different substituents and hydroxyl groups. As a consequence, a hydrogen bonding interaction gradual weakening mechanism has been perfectly verified, that is, the ESIPT reaction is more difficult to occur from MS → 5MeMS → 5FMS → 5ClMS → 5BrMS → 5MeOMS → 5AmMS molecules.


 Please wait while we load your content...
Please wait while we load your content...