Enhanced electrochemical water oxidation: the impact of nanoclusters and nanocavities†
Abstract
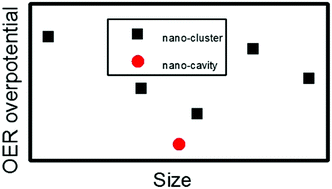
The structures of transition metal surfaces and metal oxides are commonly believed to have a significant effect on the catalytic reactions. Density functional theory calculations are therefore used in this study to investigate the oxygen evolution reaction (OER) over nanostructured, i.e. nanocluster and nanocavity, surfaces of hematite (Fe2O3). The calculated results demonstrate an optimum nanocluster size with respect to the OER overpotential. The presence of nanoclusters on the electrode is regarded as an attractive strategy for increasing the activity in photoelectrochemical water splitting. However, in this work, we found that the presence of a nanocavity is a more effective strategy for lowering the overpotential compared to nanoclusters. This finding of the nanocavity-favoured OER for hematite surfaces is verified by similar simulations of WO3 surfaces.


 Please wait while we load your content...
Please wait while we load your content...