Why pregnenolone and progesterone, two structurally similar steroids, exhibit remarkably different cocrystallization with aromatic molecules†
Abstract
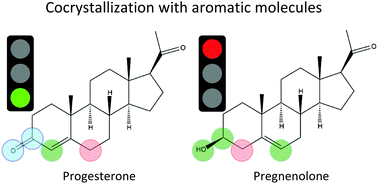
Selective binding of steroid molecules is of paramount importance for designing drugs that can target the biological pathways of only individual steroids. From this perspective, it is remarkable that progesterone (PRO) and pregnenolone (PRE), two structurally similar steroids, demonstrate a dramatically different propensity to interact with aromatic molecules. It has been recently reported that, in solid-state cocrystallization, PRO forms cocrystals with a wide variety of aromatic systems whereas PRE cocrystallizes only with a few. In this work, a simple yet effective computational procedure was developed to explain the fundamental origins of this surprising phenomenon. This procedure enables a direct comparison of the strength of intermolecular binding in the structurally similar cocrystals of PRO and PRE by generating experimentally inaccessible meta-stable cocrystals of PRE that closely resemble those observed for PRO. Direct comparative analysis shows that interactions between the α-face of the steroid and the π-electrons of aromatic molecules, the focus of previous studies, are not sufficiently different to explain the cocrystallization behavior of PRO and PRE. Instead, the observed difference is attributed to the different stabilities of the cocrystals relative to their pure components: organic and steroid crystals. It is calculated that the cocrystallization process is thermodynamically favorable in the case of PRO and unfavorable in the case of PRE. Furthermore, strong hydrogen bonds in the pure PRE crystal appear to be the major factor that makes the cocrystallization of PRE energetically unfavorable for a wide range of aromatic molecules. The fundamental analysis performed in this work has important practical implications for designing new steroid-containing crystals, selective biomolecular steroid receptors, and steroid-specific drugs. It suggests that a strategy for the selective binding of steroids should focus primarily on tuning the strength of hydrogen bonding.


 Please wait while we load your content...
Please wait while we load your content...