Concurrent aerogen bonding and lone pair/anion–π interactions in the stability of organoxenon derivatives: a combined CSD and ab initio study†
Abstract
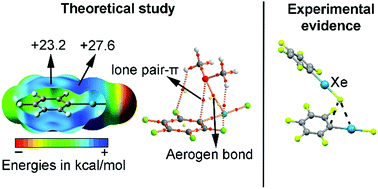
In this manuscript the ability of organoxenon fluorides to establish concurrent π-hole aerogen bonding and lone pair/anion–π interactions has been studied at the RI-MP2/def2-TZVP level of theory. The presence of both an aromatic system (benzene, trifluorobenzene and pentafluorobenzene) and a xenon atom makes these molecules suitable for simultaneously establishing both interactions. In this regard, we have used CH3CN, NH3, O(CH3)2, Cl−, CN− and BF4− as neutral and charged electron donors, respectively. Moreover, the NBO analysis showed that orbital effects contribute to the global stabilization of the complexes studied. Furthermore, we have used Bader's theory of “atoms in molecules” to analyse and characterize the noncovalent interactions described herein from a charge-density perspective. Finally, several examples retrieved from the CSD are also included, highlighting the impact of these interactions in the solid state chemistry of Xe.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...