Lysophosphatidylcholine modulates the aggregation of human islet amyloid polypeptide†
Abstract
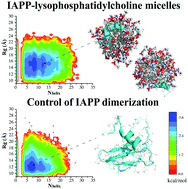
Amyloid aggregation of human islet amyloid polypeptide (IAPP) is a hallmark of type 2 diabetes (T2D), a metabolic disease and a global epidemic. Although IAPP is synthesized in pancreatic β-cells, its fibrils and plaques are found in the extracellular space indicating a causative transmembrane process. Numerous biophysical studies have revealed that cell membranes as well as model lipid vesicles promote the aggregation of amyloid-β (associated with Alzheimer's), α-synuclein (associated with Parkinson's) and IAPP, through electrostatic and hydrophobic interactions between the proteins/peptides and lipid membranes. Using a thioflavin T kinetic assay, transmission electron microscopy, circular dichroism spectroscopy, discrete molecular dynamics simulations as well as free energy calculations here we show that micellar lysophosphatidylcholine (LPC), the most abundant lysophospholipid in the blood, inhibited the amyloid aggregation of IAPP through nonspecific interactions while elevating the α-helical peptide secondary structure. This surprising finding suggests a native protective mechanism against IAPP aggregation in vivo.


 Please wait while we load your content...
Please wait while we load your content...