A dry molten globule-like intermediate during the base-induced unfolding of a multidomain protein†
Abstract
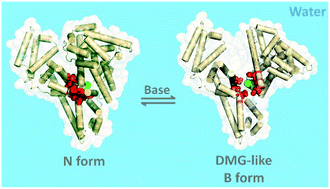
The nature of the initial structural events during the base-induced unfolding of the native (N) state of proteins is poorly understood. Combining site-specific fluorescence resonance energy transfer, size exclusion chromatography, dynamic fluorescence quenching, red-edge excitation shift and circular dichroism spectroscopy, we show here that an early intermediate during the base-induced unfolding of a multidomain protein, i.e., the B form, has features of a dry molten globule. We show that the N ⇌ B transition involves protein expansion and loosening of packing of inter-domain helices near domains I and II without the disruption of intra-domain packing or any change in hydration of the inter-domain region which resembles a molten hydrocarbon. Surprisingly, the disruption of inter-domain packing accounts for 40–45% of the total change in free energy of complete unfolding. Our results show that the disruption of van der Waals packing can be decoupled in different regions of a protein and could occur prior to hydrophobic solvation during base-induced unfolding, challenging the existing notion.


 Please wait while we load your content...
Please wait while we load your content...