Complex formation of nickel(ii) with dimethyl sulfoxide, methanol, and acetonitrile in a TFSA−-based ionic liquid of [C2mim][TFSA]†
Abstract
The thermodynamics of complex formation of Ni2+ with molecular liquids (ML), dimethyl sulfoxide (DMSO), methanol (MeOH), and acetonitrile (AN) in the ionic liquid (IL) of 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide ([C2mim][TFSA]) has been elucidated using ultraviolet (UV)-visible spectroscopy. X-ray structural analyses for single crystals grown from Ni2+–[C2mim][TFSA]–DMSO and −AN solutions at high ML contents have shown that six DMSO oxygen or AN nitrogen atoms coordinate with Ni2+ to form octahedral structures of [Ni(dmso)6](TFSA)2 and [Ni(an)6](TFSA)2, respectively. This is the same in the case of the Co2+ complex of [Co(dmso)6](TFSA)2. UV-visible spectroscopic experiments have revealed that the TFSA− anions that initially combine with Ni2+ in the IL are replaced with ML molecules in the IL–ML systems in three steps with increasing ML content. The electron donicities of the three MLs are larger in the order of DMSO > MeOH > AN. However, the stability of each complex does not simply depend on this order; the stability is higher in the order of [Ni(dmso)n] > [Ni(an)n] > [Ni(meoh)n]. In other words, the stability of the MeOH complexes is lower than that of the AN ones, despite the higher electron donicity of MeOH. The reasons for the order of the complex stabilities have been interpreted on the molecular scale, according to the stepwise enthalpies 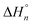 and entropies
and entropies 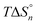 determined, together with the strength of the hydrogen bonding between the MLs and the imidazolium ring and the formation of MeOH clusters in [C2mim][TFSA].
determined, together with the strength of the hydrogen bonding between the MLs and the imidazolium ring and the formation of MeOH clusters in [C2mim][TFSA].
![Graphical abstract: Complex formation of nickel(ii) with dimethyl sulfoxide, methanol, and acetonitrile in a TFSA−-based ionic liquid of [C2mim][TFSA]](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C7CP06469A&imageInfo.ImageIdentifier.Year=2017)


 Please wait while we load your content...
Please wait while we load your content...