Water facilitates oxygen migration on gold surfaces†
Abstract
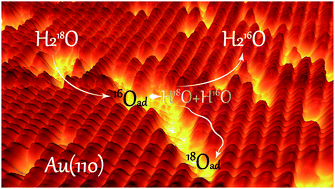
The water–oxygen–gold interface is important in many surface processes and has potential influence on heterogeneous catalysis. Herein, it is shown that water facilitates the migration of atomic oxygen on Au(110), demonstrating the dynamic nature of surface adsorption. We demonstrate this effect for the first time, using in situ scanning tunnelling microscopy (STM), temperature-programmed reaction spectroscopy (TPRS) and first-principles theoretical calculations. The dynamic interaction of water with adsorbed O maintains a high dispersion of O on the surface, potentially creating reactive transient species. At low temperature and pressure, isotopic experiments show that adsorbed oxygen on the Au(110) surface exchanges with oxygen in H218O. The presence of water modulates local electronic properties and facilitates oxygen exchange. Combining experimental results and theory, we propose that hydroxyl is transiently formed via proton transfer from the water to adsorbed oxygen. Hydroxyl groups easily recombine to regenerate water and adsorbed oxygen atoms, the net result of which is migration of the adsorbed oxygen without significant change in its overall distribution on the surface. The presence of water creates a dynamic surface where mobile surface oxygen atoms and hydroxyls are present, which can lead to a better performance of gold catalysis in oxidation reactions.


 Please wait while we load your content...
Please wait while we load your content...