Accumulation of counterions and coions evaluated by cryogenic XPS as a new tool for describing the structure of electric double layer at the silica/water interface
Abstract
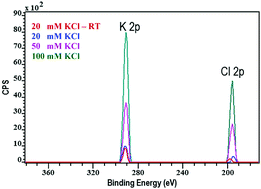
We introduce a new method of evaluating the structure of electric double layer (EDL) at the native solid/liquid interface using cryogenic X-ray photoelectron spectroscopy technique. This method is based on evaluating the atomic concentration ratio of counterions and co-ions of supporting electrolyte at the close-to-in situ state surface of colloid particles by the cryo-XPS and comparing it with analogous ratio predicted by EDL models. For silica colloids in aqueous KCl solutions at pH 6 to 8 it has been found that the latter ratio is higher than unity, as expected for the negatively charged surface of silica, but does not correspond with the prediction of the basic Gouy–Chapman EDL model for the ideal interface. However, it agrees with that deduced from experiments on electrolytic coagulation kinetics of analogous silica colloids by applying a simple EDL model of swellable ion-permeable (Donnanian) polyelectrolyte gel layer. It turns out that the traditional Stern layer-based concept of EDL at solid/liquid interfaces is not justified for metal oxides at least in KCl solutions.


 Please wait while we load your content...
Please wait while we load your content...