How do the interfacial properties of zwitterionic sulfobetaine micelles differ from those of cationic alkyl quaternary ammonium micelles? An excited state proton transfer study†
Abstract
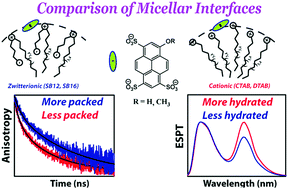
Zwitterionic surfactants (e.g. sulfobetaine), comprising both positive and negative groups in their headgroups, are essentially electro-neutral as monomers, but their micelles preferentially uptake anions similar to cationic surfactant micelles. How do the interfacial properties (e.g. interfacial hydration or headgroup packing) of a zwitterionic micelle differ from those of a cationic micelle? For this, we investigated excited-state proton transfer (ESPT) and fluorescence anisotropy decay of an interface-localized negative fluorophore, 8-hydroxypyrene-1,3,6-trisulphonate (HPTS), and its methyl analogue, 8-methoxypyrene-1,3,6-trisulfonate (MPTS), within the micellar interfaces. Two sulfobetaine surfactants (SB-12 and SB-16) and two cationic surfactants (DTAB and CTAB) with matching alkyl tails were selected for an effective comparison. The ESPT dynamics was observed to be significantly suppressed inside the zwitterionic micelle interface compared to that of the cationic micelle. This is attributed to a less hydrated interface of the zwitterionic micelles compared to that of the cationic one. Fluorescence anisotropy decay inside the zwitterionic micellar interface was also slower compared to that of the cationic one indicating a better packing of the zwitterionic surfactants at the interface.


 Please wait while we load your content...
Please wait while we load your content...