Identifying Cu(ii)–amyloid peptide binding intermediates in the early stages of aggregation by resonance Raman spectroscopy: a simulation study†
Abstract
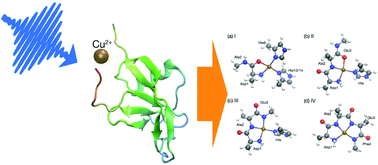
The aggregation of amyloid beta (Aβ) peptides plays a crucial role in the pathology and etiology of Alzheimer's disease. Experimental evidence shows that copper ion is an aggregation-prone species with the ability to coordinately bind to Aβ and further induce the formation of neurotoxic Aβ oligomers. However, the detailed structures of Cu(II)–Aβ complexes have not been illustrated, and the kinetics and dynamics of the Cu(II) binding are not well understood. Two Cu(II)–Aβ complexes have been proposed to exist under physiological conditions, and another two might exist at higher pH values. By using ab initio simulations for the spontaneous resonance Raman and time domain stimulated resonance Raman spectroscopy signals, we obtained the characteristic Raman vibronic features of each complex. These signals contain rich structural information with high temporal resolution, enabling the characterization of transient states during the fast Cu–Aβ binding and interconversion processes.


 Please wait while we load your content...
Please wait while we load your content...