Dynamical properties of EMIM-SCN confined in a SiO2 matrix by means of 1H NMR relaxometry
Abstract
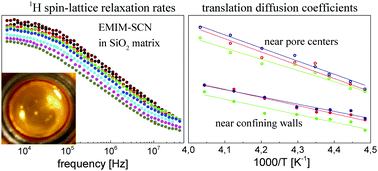
1H nuclear magnetic resonance relaxometry is applied to investigate the translational and rotational dynamics of ionogels composed of an ionic liquid (IL): 1-ethyl-3-methyl-imidazolium-thiocyanate (EMIM-SCN) confined in a nanoporous SiO2 matrix. The relaxation studies were performed in the frequency range of 4 kHz–40 MHz and the temperature range of 223–248 K for different concentrations of the IL; the ratio (no. of moles of IL/no. of moles of SiO2) yields: 1/2, 3/5 and 7/10. A thorough analysis of this large set of experimental data was performed assuming the existence of two fractions of the liquid: a core fraction (near the pore center) and a surface fraction (near the confining walls). It was shown for all concentrations that the confinement does not significantly affect the translational motion near the pore center compared to the dynamics in bulk. The diffusion coefficients in the surface fraction are considerably smaller compared to the core fraction (from one to two orders of magnitude) and the difference becomes larger with increasing temperature. The diffusion coefficients become smaller for higher concentrations – this effect is not large, but visible. Very importantly, it was shown that, despite the interactions with the surface, the diffusion in the surface fraction remains of 3D character. As far as rotational dynamics in the surface fraction is concerned, it slows down compared to the bulk (and the core fraction), but this effect is of the order of factor 2–3.


 Please wait while we load your content...
Please wait while we load your content...