Recycling of zincite (ZnO) via uptake of hydrogen halides†
Abstract
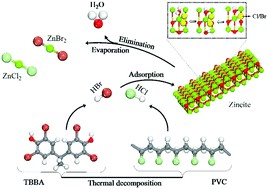
Hydrogen halides (HCl/HBr) represent major halogen fragments from the thermal decomposition of halogen laden materials, most notably PVC and brominated flame retardants (BFRs). Co-pyrolysis of halogen-containing solid waste with metal oxides is currently deployed as a mainstream strategy to treat halogen content as well as to recycle the valuable metallic fraction embedded in electric arc furnace dust (EAFD) and e-waste. However, designing an industrial-scale recycling facility necessitates accurate knowledge on mechanistic and thermo-kinetic parameters dictating the interaction between metal oxides and hydrogen halides. In this contribution, we investigate chemical interplay between HCl/HBr and zincite surfaces as a representative model for structures of zinc oxides in EAFD by using different sets of functionals, unit cell size and energy cut-off. In the first elementary step, dissociative adsorption of the HCl/HBr molecules affords oxyhalide structures (Cl/Br–Zn, H–O) via modest activation barriers. Conversion of the oxyhalide structure into zinc halides occurs through two subsequent steps, further dissociative adsorption of HCl/Br over the same surface Zn atom as well as the release of a H2O molecule. Evaporation (or desorption of zinc halide molecules) signifies a bottleneck for the overall halogenation of ZnO. Our simplified kinetic model on the HCl + ZnO system concurs very well with experimentally reported TGA weight loss profiles on two grounds: accumulation of oxyhalides until ∼700 K and desorption of ZnCl2 at higher temperatures. The thermo-kinetic and mechanistic aspects reported herein could be useful in the pursuit of a design of a large-scale catalytic upgrading unit that operates to extract valuable zinc loads from EAFD.


 Please wait while we load your content...
Please wait while we load your content...