Two-dimensional (2D) self-assembly of oligo(phenylene-ethynylene) molecules and their triangular platinum(ii) diimine complexes studied using STM†
Abstract
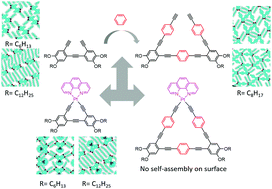
In this investigation, the two-dimensional (2D) self-assembly nanostructures of a series of cyclic oligo(phenylene-ethynylene) (OPE) molecules (L1, L2-6 and L2-12) at the 1-phenyloctane/highly oriented pyrolytic graphite (HOPG) interface were thoroughly studied using scanning tunneling microscopy (STM). Comparative STM studies with their triangular Pt(II) diimine complexes (C1, C2-6 and C2-12) were also carried out. Based on careful measurements on single molecule level STM images and density functional theory (DFT) calculations, the formation mechanisms of the nanoarrays formed were revealed.


 Please wait while we load your content...
Please wait while we load your content...