A unified understanding of the direct coordination of NO to first-transition-row metal centers in metal–ligand complexes
Abstract
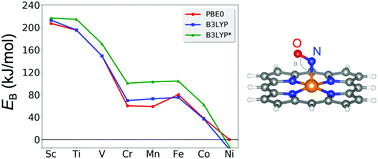
The binding of nitric oxide (NO) to heme-proteins is an important biochemical process involved in a variety of physiological functions. Here, using hybrid density-functional calculations, we systematically investigate the adsorption of NO to first-transition-row metal centers in metal–ligand complexes. Through the comparative study for different transition metal (TM) centers, we provide a unified understanding of the microscopic interactions of NO with the TM centers and related chemical trends. We found that as the atomic number of the TM center increases, the binding strength of NO is largely reduced from 207 kJ mol−1 to near zero due to the low d-orbital energies for late TM centers. The intermolecular spin coupling between the localized spins at the TM center and the NO molecule is generally antiferromagnetic, except for the case of Sc. The spin–spin coupling is determined in such a way to avoid the energy penalty associated with the electron occupation in the antibonding states of the NO-bound complex. The adsorption strength of NO is generally larger than of CO because the unpaired electron of NO occupies the associated bonding state.


 Please wait while we load your content...
Please wait while we load your content...