Chemical surface exchange of oxygen on CeO2−δ in an O2/H2O atmosphere†
Abstract
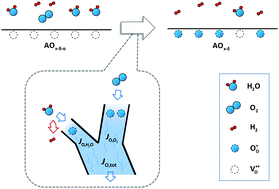
The chemical surface reaction rate constant controlling the change of oxidation state of undoped ceria, kChem, was measured at 1400 °C in the range of (∼0 ≤ (pH2O/atm) ≤ 0.163(9)) and (10−3.85 ≤ (pO2/atm) ≤ 10−2.86) via the electrical conductivity relaxation method. In humidified atmospheres, kChem is fully described as the sum of kChem,O2 and kChem,H2O, which are, respectively, the rate constants for oxidation by O2 and by H2O alone. Using measurements under appropriately controlled gas conditions, the total rate constant is found to follow the correlation kChem/cm s−1 = 10−(1.35±0.07) × (pO2/atm)0.72±0.02 + 10−(3.85±0.03) × (pH2O/atm)0.36±0.03 where the pO2 and pH2O values of relevance are explicitly those of the final gas condition. The results suggest that at such high temperatures, the concentrations of surface adsorbed species are too low to influence the independent reaction pathways.


 Please wait while we load your content...
Please wait while we load your content...