Inversion of the 11Bu and 21Ag electronic states of all-trans-1,6-diphenyl-1,3,5-hexatriene in carbon disulfide
Abstract
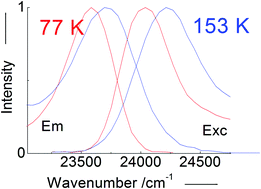
From the analysis of UV/Vis absorption spectra, emission and excitation emission spectra of solutions of all-trans-1,6-diphenyl-1,3,5-hexatriene (DPH) in carbon disulfide (CS2) within a wide range of temperatures, we can conclude that these spectra do not support the inversion of excited electronic states in DPH by an increased polarizability of the solvent as proposed by Kohler and Itoh. The proposal by Kohler and Itoh is widely accepted as an elegant demonstration of the inversion of the 11Bu and 21Ag states in the polyene DPH by a mere increase in the solvent polarizability. The evidence provided in this paper confirms, as has recently been proposed, that a ghost state as the first electronic state, 21Ag, is not necessary to explain the photophysics of DPH. The photophysics of DPH is plausibly explicable with the 11Bu state, which is directly excitable by photonic absorption from the fundamental electronic state of the compound. In this paper, we provide evidence that the UV/Vis spectra of DPH in CS2 at 77 K are most likely generated from a molecular structure of DPH constrained by solid CS2, which is clearly distinct from that present in liquid CS2.


 Please wait while we load your content...
Please wait while we load your content...