Why do the Togni reagent and some of its derivatives exist in the high-energy hypervalent iodine form? New insight into the origins of their kinetic stability
Abstract
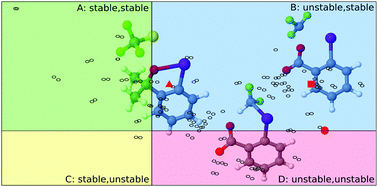
In a recent study published in ChemComm, H. F. Schaefer and coworkers showed that the Togni trifluoromethyltation reagent and some of its derivatives appear in a high-energy hypervalent form. The (kinetic) stability of these reagents is granted by the five-membered ring of their benziodoxole-based scaffold, which prevents isomerization to the (inactive) acyclic ether form. Whereas the thermodynamic stability of these reagents can be predicted on the basis of the trans influence of the electrophilic substituent, no such descriptor was found for their kinetic stability. In this study, we explore an array of Togni-type reagents, and show that the barrier to isomerization can be predicted based on the bond length between the iodine atom and the electrophilic substituent. For compounds, where this correlation does not hold, we have a reliable indication that the structure of the transition state is at variance with those in the series.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...