Multiple interaction regulated phase transition behavior of thermo-responsive copolymers containing cationic poly(ionic liquid)s†
Abstract
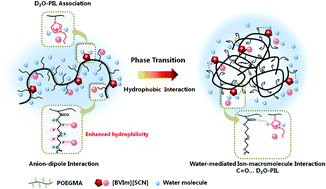
The effect of multiple interactions including anion–macromolecule interaction, water-mediated ion–macromolecule interaction and hydrophobic interaction on the phase transition behaviors of random copolymers P(OEGMA-co-BVIm[X]) comprising oligo(ethylene glycol)methacrylate (OEGMA) and imidazolium-based ionic liquids is investigated in the present study. Temperature-variable 1H NMR and FT-IR investigations demonstrated that the hydration of CH2 side chains in P(OEGMA-co-BVIm[SCN]) was enhanced due to the anion–dipole interaction between a chaotropic anion SCN− and CH2 groups, and dehydration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups served as the driving force of phase transition. In particular, the formation of C
O groups served as the driving force of phase transition. In particular, the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯D2O–PIL hydrogen bonds was preferred in P(OEGMA-co-BVIm[SCN]) during the phase transition process considering the interaction of IL–D2O associations and C
O⋯D2O–PIL hydrogen bonds was preferred in P(OEGMA-co-BVIm[SCN]) during the phase transition process considering the interaction of IL–D2O associations and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. This water-mediated ion–macromolecule interaction acted as a “linkage” among polymers, resulting in the gradual dehydration of copolymers and the formation of stable small size micelles. As for P(OEGMA-co-BVIm[NTf2]), water molecules were sequentially squeezed out of the polymer chains upon heating and the self-aggregation of polymer chains was carried out through hydrophobic interaction between OEGMA side chains with IL segments wrapped in the aggregates.
O groups. This water-mediated ion–macromolecule interaction acted as a “linkage” among polymers, resulting in the gradual dehydration of copolymers and the formation of stable small size micelles. As for P(OEGMA-co-BVIm[NTf2]), water molecules were sequentially squeezed out of the polymer chains upon heating and the self-aggregation of polymer chains was carried out through hydrophobic interaction between OEGMA side chains with IL segments wrapped in the aggregates.


 Please wait while we load your content...
Please wait while we load your content...