The reaction of fluorine atoms with methanol: yield of CH3O/CH2OH and rate constant of the reactions CH3O + CH3O and CH3O + HO2†
Abstract
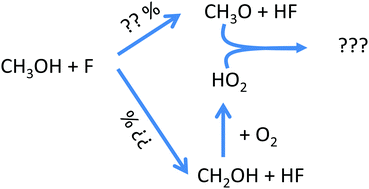
Xenondifluoride, XeF2, has been photolysed in the presence of methanol, CH3OH. Two reaction pathways are possible: F + CH3OH → CH2OH + HF and F + CH3OH → CH3O + HF. Both products, CH2OH and CH3O, will be converted to HO2 in the presence of O2. The rate constants for the reaction of both radicals with O2 differ by more than 3 orders of magnitude, which allows an unequivocal distinction between the two reactions when measuring HO2 concentrations in the presence of different O2 concentrations. The following yields have then been determined from time-resolved HO2 profiles: ϕCH2OH = (0.497 ± 0.013) and ϕCH3O = (0.503 ± 0.013). Experiments under low O2 concentrations lead to reaction mixtures containing nearly equal amounts of HO2 (converted from the first reaction) and CH3O (from the second reaction). The subsequent HO2 decays are very sensitive to the rate constants of the reaction between these two radicals and the following rate constants have been obtained: k(CH3O + CH3O) = (7.0 ± 1.4) × 10−11 cm3 s−1 and k(CH3O + HO2) = (1.1 ± 0.2) × 10−10 cm3 s−1. The latter reaction has also been theoretically investigated on the CCSD(T)//M06-2X/aug-cc-pVTZ level of theory and CH3OH + O2 have been identified as the main products. Using μVTST, a virtually pressure independent rate constant of k(CH3O + HO2) = 4.7 × 10−11 cm3 s−1 has been obtained, in good agreement with the experiment.
- This article is part of the themed collection: Bunsentagung 2018: Kinetics in the Real World


 Please wait while we load your content...
Please wait while we load your content...