A novel multimode sensor showing cation-dependent fluorescence colour†
Abstract
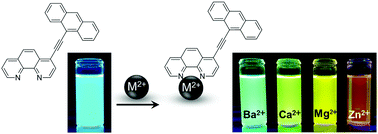
A novel sensor, 4-[2-(9-anthryl)ethynyl]-1,10-phenanthroline (1), exhibits highly intense fluorescent in the wavelength region of 440–600 nm (maximum wavelength (λf) = 470 nm) with the quantum yield (Φf) and lifetime (τf) being 0.90 and 4.2 ns, respectively, in CH3CN at 298 K. In the presence of a divalent cation (M2+ = Ba2+, Ca2+, Mg2+, or Zn2+) in CH3CN, sensor 1 can tightly bind M2+ and shows intense fluorescent (Φf = 0.90–0.19, τf = 2.1–6.9 ns) with the color being dependent on the nature of M2+ (λf = 514–584 nm). The results demonstrate that a single fluorescent sensor 1 is capable of simultaneous identification and quantitation of M2+ based on λf and the fluorescent intensity (Φf), respectively. The fluorescence maximum energy of the [1–M2+] complex is shown to correlate linearly with the pKa value of M2+. The spectroscopic and photophysical properties of sensor 1 in the absence and presence of M2+ are also discussed.


 Please wait while we load your content...
Please wait while we load your content...