Order–disorder phase transition in an anhydrous pyrazole-based proton conductor: the enhancement of electrical transport properties†
Abstract
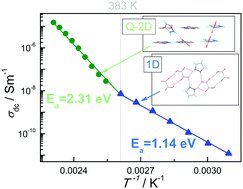
The crystal structure of 1H-pyrazol-2-ium hydrogen oxalate has been studied at 100 K. It consists of two-dimensional layers built with one-dimensional chains that contain pyrazolium and oxalate acids bonded by N–H⋯O and O–H⋯O hydrogen bonds. According to the X-ray data and the Quantum Theory of Atoms in Molecules, it was shown that weak and moderate hydrogen bonds are present in the crystal at room temperature. The thermal stability was studied with the DSC, TGA, and DTG methods: three endothermic peaks are observed at 384, 420, and 469 K. Conductivity measurements have been performed in the temperature range from 300 to 433 K. At 383 K the pyrazole–oxalic acid framework loses its rigidity and the crystal undergoes an ordered–disordered phase transition. At this temperature, the value of the activation energy of proton conductivity changes from 1.14 to 2.31 eV. The proton conduction pathways and the transport mechanism have been studied with theoretical methods.


 Please wait while we load your content...
Please wait while we load your content...