Distinct oligomerization and fibrillization dynamics of amyloid core sequences of amyloid-beta and islet amyloid polypeptide†
Abstract
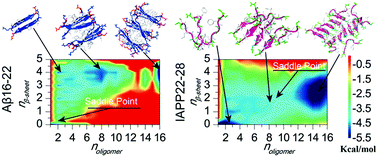
A direct observation of amyloid aggregation from isolated peptides to cross-β fibrils is crucial for understanding the nucleation-dependence process, but the corresponding macroscopic timescales impose a major computational challenge. Using rapid all-atom discrete molecular dynamics simulations, we capture the oligomerization and fibrillization dynamics of the amyloid core sequences of amyloid-β (Aβ) in Alzheimer's disease and islet amyloid polypeptide (IAPP) in type-2 diabetes, namely Aβ16–22 and IAPP22–28. Both peptides and their mixture spontaneously assemble into cross-β aggregates in silico, but follow distinct pathways. Aβ16–22 is highly aggregation-prone with a funneled free energy basin toward multi-layer β-sheet aggregates. IAPP22–28, on the other hand, features the accumulation of unstructured oligomers before the nucleation of β-sheets and growth into double-layer β-sheet aggregates. In the presence of Aβ16–22, the aggregation of IAPP22–28 is promoted by forming co-aggregated multi-layer β-sheets. Our study offers a detailed molecular insight to the long-postulated oligomerization-nucleation process in the amyloid aggregations.


 Please wait while we load your content...
Please wait while we load your content...