Theoretical study on geometric, electronic and catalytic performances of Fe dopant pairs in graphene†
Abstract
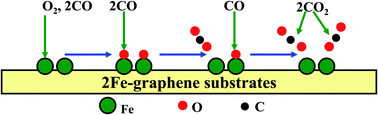
The formation geometries, electronic structures and catalytic properties of monovacancy and divacancy graphene sheets with two embedded Fe dopants (2Fe-MG and 2Fe-DG) have been systematically investigated using the first-principles calculations. It was found that the configuration of 2Fe-DG is slightly more stable than that of 2Fe-MG sheets and the two doped Fe atoms in graphene (2Fe-graphene) as active sites could regulate the stability of gas molecules. In addition, the adsorption of O2 and CO molecules could modulate the electronic and magnetic properties of 2Fe-graphene systems. Moreover, the adsorption behaviors of reactants could determine the reaction pathway and energy barrier of the catalytic oxidation of CO. On the 2Fe-graphene substrates, the adsorptive decomposition of O2 molecules (<0.20 eV) and the subsequent Eley–Rideal (ER) reaction (2Oads + 2CO → CO2) (<0.60 eV) have low energy barriers. In comparison, the CO3 complex is quite stable and its formation needs to overcome a higher energy barrier (>0.90 eV). Hence, the dissociation of O2 as an initial step is an energetically more favored process. These results provide valuable guidance for the design of functionalized graphene-based devices.


 Please wait while we load your content...
Please wait while we load your content...