Planarization of B20 clusters by Si and C atom substitution
Abstract
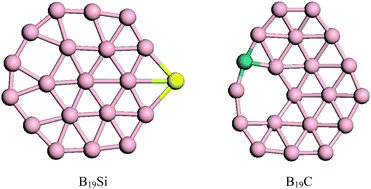
An optimization strategy combining a global semi-empirical quantum mechanical search and all-electron density functional theory was adopted to determine the lowest energy structures of B19Si and B19C clusters. The planarization of a B20 cluster by Si and C atom substitution was observed. The structural transition was from the double-ring tubular B20 to an almost perfect planar B19Si and a quasi-planar bowl B19C. B19Si possessed a geometry with a central B atom surrounded by a six-membered ring and a 13-atom outer ring. B19C adopted a geometry with a B5C six-membered hole. Both Si and C atoms occupied peripheral positions. The observed planarization may be attributed to sp2 hybridization, changes in the peripheral bonding, and structural mechanics. Some properties, including the HOMO–LUMO gaps, on-site charge on Si and C atoms, and deformed charge distribution, were discussed.


 Please wait while we load your content...
Please wait while we load your content...